Oral argument December 23 2023, Harrington v. Purdue Pharma
Court conflicted over Purdue Pharma bankruptcy plan that shields Sacklers from liability, SCOTUSblog, Amy Howe.
Conflicted? There is nothing to be conflicted about with Purdue and the US Opioid Epidemic. As was pointed out repeatedly. The Supporting Facts Leading to the Cause of the Opioid Epidemic is pretty simple. Purdue promoted the use of opioids claiming it was nonaddictive. Indeed the usage was promoted by a simple one paragraph letter published in the 1980 NEJM and used improperly to convince doctors and people the uses of OxyContin, etc. was nonaddictive.
As taken from A 1980 Letter on the Risk of Opioid Addiction | NEJM, June 1, 2017.
The result of the misuse of that brief paragraph was phenomenal. The prescribing of opioids such as oxycodone increased dramatically in the United States and Canada over the past two decades.1 From 1999 through 2015, more than 183,000 deaths from prescription opioids were reported in the United States,2 and millions of Americans are now addicted to opioids. The crisis arose in part because physicians were told the risk of addiction was low when opioids were prescribed for chronic pain.
A one-paragraph letter that was published in the Journal in 19803 was widely invoked in support of this claim, even though no evidence was provided by the correspondents (see Section 1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). The manufacturer of oxycondone used this letter to promote the sales and use of opioids. The letter itself was sent to the NEJM in 1980 by Doctors Jane Porter and Herschel Jick covering a Boston Collaborative Drug Surveillance Program at Boston University Medical Center, Waltham, MA. The published letter said this:
“Recently, we examined our current files to determine the incidence of narcotic addiction in 39,946 hospitalized medical patients who were monitored consecutively. Although there were 11,882 patients who received at least one narcotic preparation, there were only four cases of reasonably well documented addiction in patients who had no history of addiction. The addiction was considered major in only one instance. The drugs implicated were meperidine in two patients, Percodan in one, and hydromorphone in one. We conclude that despite widespread use of narcotic drugs in hospitals, the development of addiction is rare in medical patients with no history of addiction.”
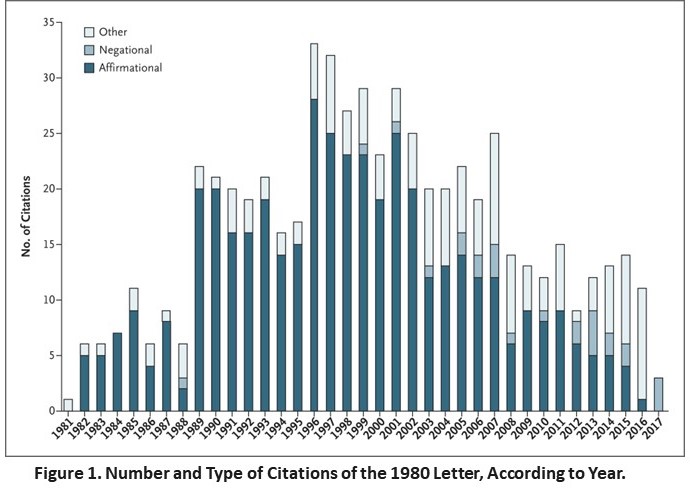
In turn this simple letter was used any number of times to promote the use. We identified 608 citations of the index publication and noted a sizable increase corresponding with the introduction of OxyContin (a long-acting formulation of oxycodone) in 1995 (Figure 1). Of the articles that included a reference to the 1980 letter, the authors of 439 (72.2%) cited it as evidence that addiction was rare in patients treated with opioids. Of the 608 articles, the authors of 491 articles (80.8%) did not note that the patients who were described in the letter were hospitalized at the time they received the prescription, whereas some authors grossly misrepresented the conclusions of the letter (Section 3 in the Supplementary Appendix). Of note, affirmational citations have become much less common in recent years. In contrast to the 1980 correspondence, 11 stand-alone letters that were published contemporaneously by the Journal were cited a median of 11 times.
I laid the groundwork now back to SCOTUS chatting with Purdue’s representatives. Why this is in SCOTUS puzzles me. It is pretty well known the sale of OxyContin was done in a fraudulent manner. The misuse of the Jick and Porter letter used numerous times to ensure healthcare and patients there was no risk of addiction. I am puzzled why the company has a venue in SCOTUS. This was a slam dunk for the victims and the public. Here is some of the back and forth in court.
~~~~~~~~
Justice Elena Kagan also appeared skeptical of the government’s position. She observed that the support for the plan is “overwhelming,” “among people who have no love for the Sacklers, who pretty much think the Sacklers are the worst people on earth.” And although the government’s argument, in her view, is based on “highfalutin principles of bankruptcy law,” another principle of bankruptcy law is the obligation to maximize the value of the bankruptcy estate. Here, she emphasized, a “huge, huge, huge majority” of the creditors and victims have decided that if the current plan isn’t confirmed, they won’t receive anything.
Jackson took a less sympathetic view of the plan. Even if releases like the ones that shield the Sacklers could generally be authorized, she asked, why should we do it in this case? The reason why Purdue Pharma does not have money to pay its creditors, she said, is because the Sacklers took money out of the company for themselves.
Garre responded that of the $11 billion that the Sacklers took out of the company in the years leading up to the bankruptcy, 40% went to pay taxes, so that 97% of the post-tax funds transferred to the Sacklers would in fact be included in the settlement. The bankruptcy court, he said, made careful findings that the Sacklers’ contribution was best possible one available for the victims. If the plan doesn’t go forward, he added, there would be “serious issues” for the victims in trying to collect any award that they might receive in a lawsuit.
Jackson remained skeptical, telling Garre that any collection problems could be attributed to the fact that the Sacklers have taken the money out of the United States.
Justice Amy Coney Barrett also appeared unconvinced. She noted (as did Gorsuch) that if the Sacklers filed for bankruptcy, they would not be shielded from liability for claims alleging fraud. Some justices questioned whether the federal government has the right to challenge the confirmation of the plan at all. Justice Clarence Thomas asked Gannon what the interest of the U.S. Trustee, the Department of Justice official appointed by the attorney general to oversee bankruptcy cases, has in “undoing” the Purdue Pharma plan when the overwhelming majority of creditors and victims want to have their claims against the company and the Sacklers resolved.
Gannon responded that the U.S. Trustee plays a “watchdog” role. Congress, he explained, gave the U.S. Trustee the power to “raise” and “be heard” on any issue, which is precisely what the government is doing in this case. The presence of the U.S. Trustee, Gannon added, “helps to ensure that there is a disinterested observer.
Gannon’s response did not mollify Thomas or Kavanaugh, who told Garre that he had a “strong argument” that the U.S. Trustee did not have a right to challenge the plan. But, Kavanaugh asked, what about Ellen Isaacs, whose son died of an opioid overdose, and opposes the bankruptcy deal.
Garre pushed back against any suggestion that Isaacs would have a right to sue, noting that she had not identified a claim based on Purdue Pharma’s conduct that would be affected by the plan. Moreover, he noted, it would be unusual to rely on the standing of a party, like Isaacs, who had not addressed the legal question presented in the case in her brief.
Barrett, however, saw the federal government’s position differently, characterizing the U.S. Trustee as representing the “invisible debtors” in the case.
~~~~~~~~
What happened in SCOTUS is a pretty interesting read. Purdue is not home free yet. Some of the conservative justices were skeptical. I suspect this may be the court swatting Purdue and the Sacklers.
A 1980 Letter on the Risk of Opioid Addiction | NEJM
Doctor, Who Was Paid by Purdue to Push Opioids, Will Testify Against Drugmaker Angry Bear
Who is Deciding How to Spend $50 Billion in Opioid Settlement Cash? Angry Bear
U.S. Seeks to Block Bankruptcy Plan That Would Free Sacklers From Opioid Claims, Angry Bear
Is ‘Big Pharma’ To Blame For the Opioid Crisis? – Angry Bear
Purdue Offers Up $10 – 12 Billion to Settle All Lawsuits – MedPage Update – Angry Bear

The ratio of people who died from opioid overdose for a proven pain diagnosis is trace compared to people who died from recreational use. Recreational users pile on multiple addictions to determine their fate. Many senior citizens suffer everyday and are prevented from having a full life because of this farce.
Bill,
I think the main reason the case is before the court is the question of the Bankruptcy Court’s power to grant the Sacklers a release when they are not before the court as bankruptcy applicants. It’s not so much on the merits of the proposed settlement. Individual claimants are seeking to preserve their rights to pursue the Sacklers even if Purdue is released. The Purdue release would be appropriate inasmuch as it is the bankruptcy applicant. The problem is that the Sacklers are offering personal money to the settlement amount but will not do so without a release of their potential liability. Their money is deemed necessary by the bankruptcy claimants to settle the claims. In effect the Sacklers are seeking to cut off the rights of dissenting claimants to opt out of the class settlement in the bankruptcy action as to Sacklers’ personal liability.
Jack:
The evidence going back years to when Oxycodone was introduced in 1995 was that it was non-addictive. They lied year after year of it being nonaddictive and did so even after one court determined their company executives lied earlier. I have multiple posts on Angry Bear covering the Sacklers and Purdue. They lied. How do you square it Jack?
The Sacklers have no basis to cut off the dissenting ones based upon the others receiving some type of settlement. They are playing one against the other,
Yes, and the question is whether or not the Supreme Court will let them do that.
Dissenting claimants are riding coattails of State lawsuits. I have not seen any use of the settlement money to help seniors who have proven pain diagnosis after 50 years of physical demanding jobs. They are being denied a full life. Medicare would save billions treating seniors and veterans with a controlled daily or no more than a few days dispense of the medications to keep them out of the hands of recreational users. Recreational users was the core problem. It anyone deserves litigation. it is them.