Big Pharma Influence in State, Federal Government, and Everyday Life
How Pharma Influences Legislation They Do Not Like
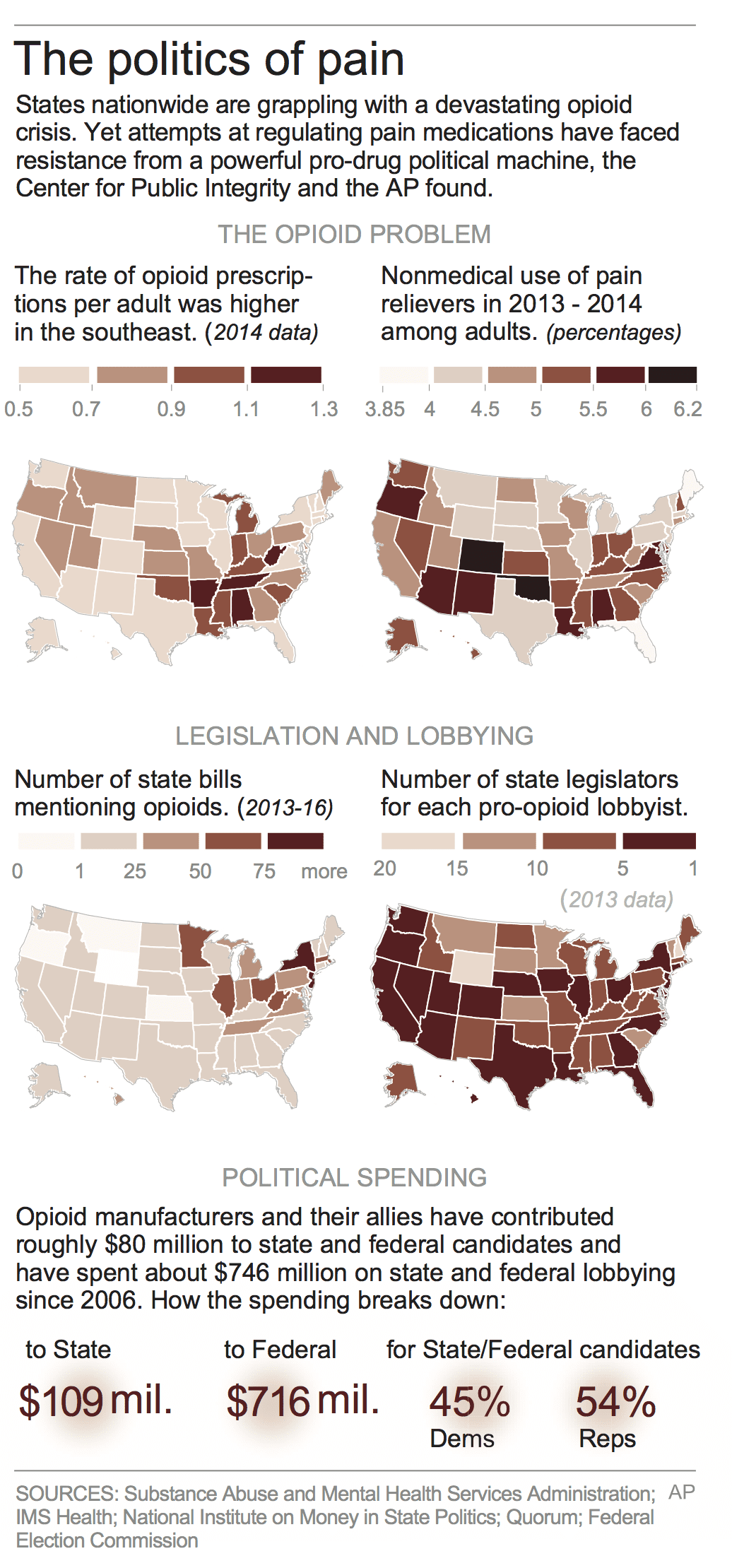
From 2006 to 2015, pharmaceutical companies spent $880 million in lobbying state and federal legislatures and contributing to campaigns to prevent laws restricting Opioid prescriptions. Their lobbying expenditures has outstripped those advocating for greater controls on prescriptions by 200 times giving them greater influence at the state level.
The pharmaceutical industry influences more than just drug developments. They can have a hand in key legislation as well. https://t.co/ecEnVFB9XZ
— ConsumerSafety (@ConsumerSafetyO) February 22, 2018
In 2015, 227 million prescriptions were written for opioids such as OxyContin, Vicodin, and Fentanyl. This was enough prescriptions to give nine of ten adults a bottle of pills. With its aggressive lobbying, the pharmaceutical industry maintains the “status quo of aggressive prescribing of opioids and reaping enormous profits” according to Dr. Andrew Kolodny of Physicians for Responsible Opioid Prescribing.
Opioids are used to interact with the receptors (protein molecule that receive chemical signals from outside a cell causing a cellular/tissue response) in the body. They work with sites in the brain and nervous system known as opioid receptors to stop pain messages from reaching the brain effectively telling you to not feel pain. Opioids are used to counter pain from injuries, surgery, and chronic pain.
While campaigning for a law limiting the initial prescription quantity of an Opioid, Jennifer Weiss-Burke came to realize the strength of the pharmaceutical lobby in New Mexico. Her son Cameron was sent home with a bottle of Percocet to combat and dull the pain from a broken collar bone. A few years later, Cameron died of a heroin over dose.
Jennifer Weiss-Burke had lobbied the New Mexico legislature to limit “initial” pain-killing opioid prescriptions to seven days outside of the needs of chronic pain patients. The bill died in the New Mexico legislature. There was no open discussion of its merits, suggested improvements, proposed compromises, or discussed safeguards to protect those using Opioids for chronic pain. Instead, the Pharmaceutical Lobbyists privately called on each legislator to discuss why they should oppose the bill Weiss-Burke was advocating.
One of the manifestations of substance abuse in New Mexico was prescription drug overdose death. It was not a significant problem ten years before; but, it was developing and unidentified as a significant problem. In 2011, Cameron Weiss Burke died of a heroin overdose. Between 1992 and 2013, New Mexico was at or near the top for drug overdose deaths nationwide. In 2013, New Mexico had a drug overdose death rate of ~ 22 per 100,000 ranking third nationally behind West Virginia and Kentucky (CDC). Yet, the New Mexico legislature chose not to react to this plague and take action?
The pharmaceutical industry is profitable and wields influence across both sides of the aisle in
Congress and at the state level. In the past its lobbying efforts paid off with Congress blocking Medicare, Part D, and the ACA from negotiating directly with Pharma on pricing. Just how strong and profitable is pharmaceuticals? As Dr. Perry Wilson explains; “Different chronic diseases have different patterns of price increases. The biggest increase was seen in diabetes care (1993 – 2013) and driven largely by the rising prices of pharmaceuticals” of which the cost of manufacturing did not increase. Now Secretary of HHS, Alex Azar as the CEO of Eli Lilly raised the price of the century old drug Humalog used to treat diabetes by 345% taking it from $2,657.88 per year to $9,172.80 per year.
With greater profitability comes the ability to employ lobbyists who can call on state and congressional legislators. The Opioid industry, associated companies, and their allies have contributed to many candidates at state-level offices and employ an army of lobbyists covering the 50 state legislatures. From 2006 to 2015, the Pain Care Forum, a loose coalition of drugmakers, trade groups and dozens of nonprofits supported by industry funding, spent $740 million to stop laws governing the prescription of opioids. Up from 2016, the pharmaceutical and health products industry spent a record $78 million in 2016.
Because of their lobbying strength in Congress and states, laws and regulations are skewed to benefit the pharmaceutical industry. Medicare, Part D, and the ACA are blocked from negotiating directly with Pharma while insurance companies are too small to take on Pharma and often resort to using Medicare pricing plus a markup. With the appointment of nominee for HHS secretary Alex Azar, the environment for lower pharmaceutical costs by allowing Part D, Medicare, and the ACA to negotiate directly looks dim.
What If They Like a Piece of Legislation?
The pharmaceutical industry can block state legislation by lobbying. If they like a piece of legislation, they can also lobby for it. Fifty-eight pharmaceutical and 26 biotech companies spent $192 million to push the 21st Century Cures Act bill through Congress in 2016. The bill potentially saves drug and device companies $billions in bringing products to market. The Food and Drug Administration will have new authority and tools to speed up approvals and shortcut the process.
– Medical schools, hospitals, and doctors will see increased research dollars coming from a $4.8 billion windfall to the NIH biomedical research organization subject to annual appropriations. “60 schools, 36 hospitals, and several dozen groups representing physician organizations” spent $120 million in lobbying efforts.
– Over two years, $1 billion in state grants will be spent to address Opioid abuse and addiction most of it going to treatment. Mental health and Research got a boost with $millions for old and new programs. Mental health, psychology, and psychiatry groups spent $1.8 million in lobbying disclosures including the 21st Century Cures bill.
– Specialty disease and patient advocacy groups supported the legislation and lobbied Congress. Many of these groups get a portion of their funding from drug and device companies. The bill includes more patient input in the drug development and approval process. The passage of the bill shows off the clout of such groups in their spending of $6.4 million in lobbying.
– More than a dozen computer, software and telecom companies also kicked in for Cures Act lobbying totaling $35 Million in lobbying on the Cures Act as well as other legislation. It will benefit hospitals and groups requiring new technology and programs.
– Funding for the giveaways? The ACA lost $3.5 billion or 30% of its funding taken from its Prevention and Public Health Fund established to promote the prevention of Alzheimer’s disease, hospital acquired infections, chronic illnesses and other ailments.
The bill does nothing to address the rising cost of pharmaceuticals. It promotes unproven and unsafe drug and device approvals which groups such as Public Citizen, Consumer Safety Org., The Associated Press, The Center for Public Integrity, and the National Center for Health Research fought against. The bill leaves the FDA under funded with a miserly boost of $500 million through 2026 to take care of the additional priorities imposed upon the FDA.
And The Politics?
Michigan Representative Fred Upton’s sponsored 21st Century Cures which will save drug and device companies $billions in bringing products to market by short circuiting FDA testing. The Food and Drug Administration will have new authority and tools to bypass studies and speed up approvals. Translated what this means is; the bill allows the FDA to approve new uses, or indications, for existing drugs without rigorous clinical trials and tests now being conducted following proven practices. This would include not taking randomized samples to prove the new drugs are safe and effective. Instead, the FDA could rely on “real world evidence,” which includes observations, safety and side-effect claims, and other data not subject to rigorous analysis. According to Public Citizen’s Michael Carome this a much lower level of evidenced and proven usage.
No friend to the ACA, Michigan Representative Fred Upton sabotaged the ACA Rick Corridor Program and continues to weaken it by taking $3.5 billion or 30 percent of the funding from the Prevention and Public Health Fund to fund the 21st Century Cures Act, a continuation of the Republican attack on the ACA. Since 2000, Representative Fred Upton has received pharmaceutical contributions in excess of $1 million according to Open Secrets Org.
Besides weakening the testing of new pharmaceuticals, the 21st Century Cures does “nothing” to fight the rising cost of pharmaceuticals. Henry Azar took Eli Lilly’s Humalog diabetes drug and increased the pricing of it from $2,657.88 per year to $9,172.80 per year. After acquiring Vimovo, Horizon Pharma increased the price of it from $138 to $2,979 per 60-pill bottle. This is not the end of the story with Horizon. They will sell to customers at a much lower price and bill insurance at the higher price. Turing Pharmaceuticals Martin Shkreli bought the rights to the 62 year old drug Daraprim and immediately increased the price for it from $13.50 to $750 a pill. The same as other drugs, EpiPens will also rise and fall in price at the discretion of its manufacturer with little regard for actual cost.
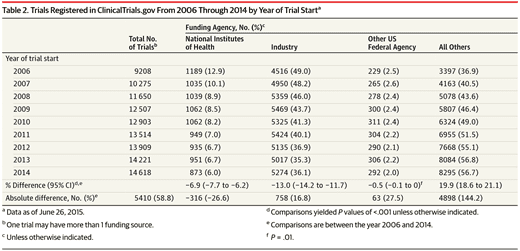
In the December 15, 2015 JAMA publication, an article (Industry-financed Clinical Trials on the Rise as the Number of NIH-funded Trials Falls) by Stephan Ehrhardt, MD, MPH1; Lawrence J. Appel, MD, MPH2; Curtis L. Meinert, PhD suggests a growing influence of clinical trials conducted by companies with a vested interest in the outcome and a dilution of the impact of government-funded trials. “The number of newly registered trials doubled from 9,321 in 2006 to 18,400 in 2014 (Table 1). The number of industry-funded trials increased by 1965 (43%). Concurrently, the number of NIH-funded trials decreased by 328 (24%).”
While results from NIH-funded clinical trials are more apt to provide the basis for prevention and treatment recommendations; industry-backed studies can produce biased results leading to an increased concern for patient safety. Such was the case with Johnson & Johnson and Bayer’s anticoagulant Xarelto. Data was withheld during its industry-sponsored trial that would have deemed it less safe than traditional warfarin. It was approved without an antidote to bleeding complications in 2011. Thousands of Xarelto lawsuits have been filed against the manufacturer because of the medication’s severe internal bleeding complications.
NIH trials are down by 24% and commercial trials are up 43% many (71%) of which were also foreign funded. Funding for the NIH has fallen 14% since 2006 when adjusted for inflation and will decrease more as adequate funding has not been provided for the 21st Century Cures Act.
In a letter to Democrats Reid and Pelosi from various organizations, it was asked the 21st Century Cure Act be delayed until such time as rising prescription prices are addressed. “ Moving forward with this legislation now would be a missed opportunity to address unaffordable prescription drug prices. There is no justification for moving forward with legislation providing substantial benefits to the drug industry without achieving something in return.”
Again, pharmaceutical company lobbying Congress plus efforts by people such as Michigan’s Rep. Fred Upton, has helped the industry reap substantial government sponsored benefits with little reciprocation in lower pricing. Michigan Representative Fred Upton was also the recipient of $597,000 from the Pharmaceutical/Healthcare Industry from 2014 into 2018. The initial bill was started in 2014.
With the 21st Century Cures Act underfunding of the NIH, increased commercial funding of clinical trials, and the relaxing of the need for clinical trials; can the pharmaceutical industry be counted on to be diligent in its testing of new products using “a lower level of evidence than the gold standard, which is randomized controlled trials” (Public Citizen’s Michael Carome)?
It appears the provisions of the 21st Century Cures Act are another stepping stone leading to the erosion of the ability of the FDA to certify the safety of new drugs and their manufacture which began in 1992 with the Prescription Drug User Fee Act (PDUFA). This allowed pharmaceutical companies to pay user fees to the agency in exchange for speedier reviews of their products. The new fees comprise 42% of the FDA’s budget today having grown from 8.5% in 1997. Companies would not be paying these fees if there were not a return for the cost in the market.
One might not think this would increase the danger the safety of new drugs; however, the 10-month time limit assessed does have its negative results. “For every 10 month of reduced review time, there is a correlated 18% increase in serious adverse reactions, 11% increase in hospitalizations, and 7% increase in deaths related to an approved drug.”
How Pharma Avoids Following the Law and the Penalties
The cost of doing business in pharmaceuticals includes paying the fines resulting from getting caught promoting drugs improperly or illegally. There are many prescriptions being written for Opioids for which there are alternate treatment strategies. When challenged, the industry and spokes people are quick to react to any calling for different strategies or limitations of usage.
For example, in 2001 Pfizer’s was ready to put Bextra on the market as a new class of painkillers known as Cox-2 inhibitors, supposedly safer than generic drugs, many times more expensive as ibuprofen, and translating into higher profits. Late in 2001, the FDA placed a qualifier on the use of Bextra for patients with extreme pain. The FDA found it was not safe for people with a risk of heart attacks or strokes and limited the usage of Bextra to people with arthritis and menstrual cramps.
This did not stop Pfizer and its partner Pharmacia from marketing Bextra as safe to use up to a 40 mg dosage. The same as what many healthcare insurance companies do with using doctors for approval of prescription, the companies hired expert doctors. Except these doctors in sales were to call on and convince other doctors (customers) of the safety of Bextra. After all, who argues with a doctor of medicine? Pfizer and its soon to be owned partner pushed the envelope with their claims of safe usage.
In the end what prevented Pfizer from a severe punishment was its size and the many other Pfizer drugs sold to and used by Medicare, Medicaid, and more then likely the VA. “Any company convicted of a major health care fraud is automatically excluded from Medicare and Medicaid. Convicting Pfizer on Bextra would prevent the company from billing federal health programs for any of its products. It would be a corporate death sentence.” The punishment stopped at fines for the illegal selling of a dangerous drug with Pfizer, again a TBTF or Too Big To Ban scenario? To comply with the law; the newly incorporated Pfizer subsidiary Pharmacia & Upjohn Co. Inc., on the same day prosecutors and Pfizer agreed Pharmacia would plead guilty, was banned from billing Medicare or Medicaid. Pfizer continued to do business as normal. Pharmacia & Upjohn Co. Inc. took the fall not once for Pfizer but twice and Pfizer walked away unscathed except for a fine.
By run75441 (Bill H)

“In the past its lobbying efforts paid off with Congress blocking Medicare, Part D, and the ACA from negotiating directly with Pharma on pricing.”
Let me rephrase that for you. “In the past accepting payments from pharma lobbyists resulted in Congress voting against the public interest on Medicare, Part D, and the ACA from negotiating directly with Pharma on pricing. “
Thorough and well written run
I founded STOPPNow (Stop the Organized Pill Pushers) due to the increasing numbers of drug addicted babies I was caring for as a neonatal nurse at Joe DiMaggio Children’s Hospital in Broward County Florida.
In 2010, 98% of the top dispensing physicians in our nation were in Florida. There were 150 “pill mills” in the one county of Broward with others scattered throughout the state. At that time there were 7 deaths/day in Florida. STOPPNow began holding peaceful protests in front of the pill mills to draw attention. The letters I was writing to Tallahassee were having no effect. I was in Marsha Blackburn’s office in Washington DC 2014 prior to passage of the bill that tied the hands of the DEA as seen on 60 Minutes/Washington Post and in the book STOPPNow. I shared the court documents DEA vs Walgreens and asked they not co-sponsor the bill with Marino. And of course the bill was passed. All we have is DEA and law enforcement in this fight.
Drug addicted babies have increased by 1000% in Florida over the last decade. And the deaths from opiates continue to escalate. I do see a change occuring though. Last session in Tallahassee Rep Pigman amended a bill to include limited opiate prescribing. It passed the house. Senator Jeff Clemens of WPB deleted that portion of the bill on Good Friday at 3:47 pm. Our govenor showed no support for the passage of the limited opiate prescribing. This session Govenor Scott does support limited opiate prescribing we have a bill S 8 that has passed all committees unopposed and is on the Senate Floor now. I believe this will pass and we can curtail new addictions. my website stoppnow.com tab current projects shows what we need help working on. Next I will be working on a bill in Washington (Senator Joe Manchin) to rescind the bill passed by Marino/Blackburn and give DEA back authority to go after rogue pharmacies and doctors. STOPPNow reveals the corruption and greed that continue to fuel the opiate epidemic. It is a first-hand account of the opiate epidemic.