Covid Vaccination one dose or 2 III
First, I want to discuss the evidence on effectiveness of the first dose of the BioNTech Pfizer Covid 19 vaccine. The data from few person – weeks of the phase III trial suggested that one dose was highly effective after 10 days. This is based on 4 of roughly 40 cases in vaccinated participants.
Much larger samples from the not randomized controlled just by the general population experience in Israel suggest lower effectiveness. I think the most interesting data are from the Israeli HMO Maccabi who followed a cohort of 50,777 vaccinated Maccabi members. The plot shows 7 day moving averages of diagnosis by PCR. They are very close for vaccinated and not vaccinated Maccabi members for about 14 days. This suggests that all of the many biases from the non randomized trial roughly cancel out. Then after 14 days they diverge ending up with a rate of infection among the vaccinated about 35% that of the unvaccinated.
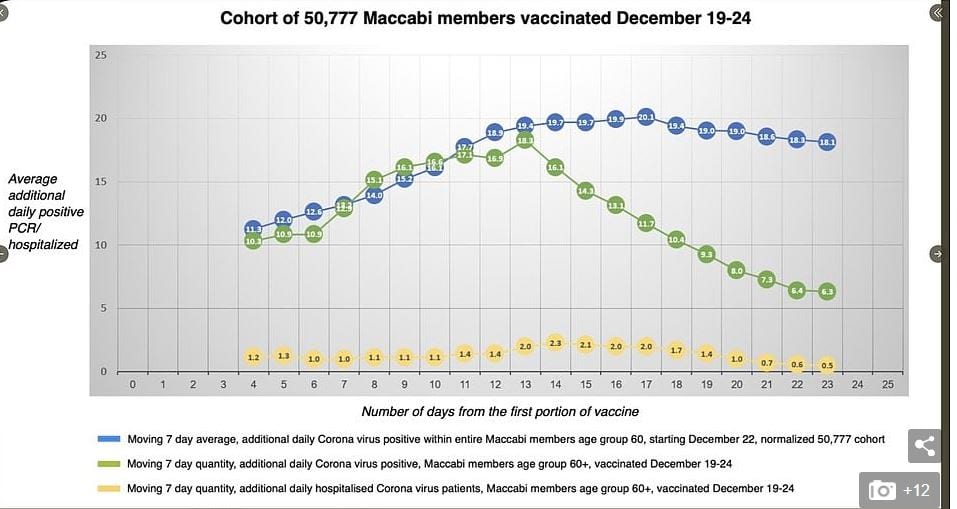
Then they gave the second dose. Ten days later, the rate of infection among the twice vaccinated was very low
Anat Ekka Zohar, a vaccine statistics analyst at Israel’s Maccabi Healthcare Services, told The Times of Israel on Thursday that the Pfizer vaccine so far has been 92% effective in Israel. The data comes from a group of 163,000 Israelis tested 10 days after their two-dose regimen.
If one is willing to extrapolate from the moving average centered on day 21 or so, then it appears that the first dose has a larger beneficial effect than the second — 60 some % not the additional 30 some %. However, there remains no evidence on the durability of the effect of the first shot.
The UK is sticking with a long delay between shots. Other countries are sticking with the 3 week interval used in the trials.
I think a key consideration is mutations which reduce the affinity of antibodies to the virus particles (in particular the mutation of amino acid 484 isolated in South Africa and Brazil). A level of blocking antibodies sufficient to protect against the now prevalent strain, might not protect against the mutant strain. This means that a weak vaccine would tend to select the mutant strain. With antibiotic treatment of bacterial diseases it is agreed that thorough treatment (a full course of antibiotics) is needed to avoid selecting resistant strains; this is especially important if multiple mutations each confers partial resistance.
There is (nonpeer reviewed) evidence that the Pfizer vaccine neutralizes virus particles bearing the key known mutations.
We engineered three SARS-CoV-2 viruses containing key spike mutations from the newly emerged United Kingdom (UK) and South African (SA) variants: N501Y from UK and SA; 69/70-deletion+N501Y+D614G from UK; and E484K+N501Y+D614G from SA. Neutralization geometric mean titers (GMTs) of twenty BTN162b2 vaccine-elicited human sera against the three mutant viruses were 0.81- to 1.46-fold of the GMTs against parental virus, indicating small effects of these mutations on neutralization by sera elicited by two BNT162b2 doses.
I think this tends to support the non UK approach of sticking to 2 doses 3 weeks apart not the UK approach (which I advocated before they did it) but I sure am sure that I am not sure.

I agree this raises some questions about delaying second doses. A few additional thoughts:
This is just Pfizer. Even if we agree that Pfizer doses should not be delayed, we might want to delay second Moderna doses.
The end point of this data is positive covid pcr tests, not covid symptoms (used in the trial). This might suggest that one shot is better at reducing illness than transmission through asymptomatic infection. I’m not sure how to think about this.
Even if we buy the 60% efficacy, 2 times 60% is still more than 1 times 95%. This still argues for delaying, though not as much 80% to 90% efficacy of the first dose.
The 60% figure I think is biased down by the cutoff at the second shot if immunity builds over time. This argues for delaying second doses.
A reasonable case could be made for delaying first doses until all the most vulnerable people are vaccinated, then giving second doses to the most vulnerable before moving on to lower priority groups. One strong objection to this approach is just logistical complexity.
Whatever we do, we need to track results and be prepared to change course as the data comes in. Remember Keynes! Figuring out the best way to do this with observational data is critical. Remember that a significant number of people simply won’t show up for their second doses. We should try to learn from their experiences. Although it will probably take too long to help in the critical next few weeks.
Here is a link to the pre-peer reviewed paper by the Maccabi group. It discusses some possible explanations for differences from the clinical trial data.
https://www.medrxiv.org/content/10.1101/2021.01.27.21250612v1
There have been a lot of tough choices. In VA we have only recently overtaken the supply with effective logistics for vaccination. Most importantly though has been the prioritization. First the front line healthcare workers and the institutionalized. VA has only just begun to vaccinate school teachers and other face to face contact workers. Those that can work from home go to the back of the line unless over 75 or immune system compromised like organ transplant patients.
The supply problem should be resolved soon now. OTOH, rolling up our sleeves for an outdoor shooting gallery during a blizzard remains a challenge.
Maybe I’m misreading something here, but doesn’t the green line in the graph show a sharp and steady decline in the incidence of virus positives in the selected group starting 13 days from the initial vaccination and continuing for the 9 or 10 following days shown in the graph? Presumably, nobody received a second dose early enough to create any additional immunity that would show on the graph, which shows about a 2/3 reduction in positive test results by the time when the second shot was supposed to be given. Is there any data from this database that shows any benefit from a second dose, given after 3 weeks, within the time period for which such data might already exist?